WP1
SofTMech Work Package WP1 Progress Report
L. Mason, N. Qi, X. Zhuang, R. Ogden, M. Olson, H. Yin
July 2021
1.1 Mechanical model of whole cell
Work done so far (theoretical)
The number of elements within the cell has now been extended to include stress fibres, the cell membrane and the nucleus, with material properties given for each element in addition to those of the F-actin cortex and soft neo-Hookean core. This has all been implemented in FEAP and specific calculations that account for the effects of the individual components on the overall response of the cell are in progress.
A separate project, which links with WP5, has developed a growth/remodelling model for the myocardium based on the analysis of growth relative to the deformed configuration as distinct from the (unattainable) so-called virtual stress-free configuration. When specialized to the latter, the results agree with those obtained by Kuhl et al. (2010) [Göktepe S, Abilez OJ, Kuhl E. A generic approach towards finite growth with examples of athlete's heart, cardiac dilation, and cardiac wall thickening. J Mech Phys Solids, 2010;58:1661-1680]. On the other hand, by using the more realistic deformed configuration with its residual stress as the basis for growth, significantly different results are obtained. A substantial paper on this topic has recently been submitted for publication. In August 2020 Yangkun Du was appointed as a Post. Doc to continue the theoretical development of the single cell model
Our plans for the next several months are:
With the grant coming to an end on the 31st July, 2021 Dr. Yangkun Du will move to an RA position with SofTMechMP to provide continuity as the new Centre-to-Centre collaboration moves forward.
Work done so far (experimental)
Within the experimental programme, we have developed a novel method to measure the viscoelastic property of a cell and demonstrated its use as a potential “mechanical biomarker” to indicate the ability of cancer cell invasion. The results have been published [1]. In another study which is associated with WP2 and WP6, we have established an AFM method for studying the interactions between cell and cell matrix. This work has already contributed to the discovery of a highly pro-invasive extracellular matrix deposition by mutant p53 tumor cells [2].
With these capabilities, we have investigated how alternation of cytoskeleton by MAPK signalling pathway can reduce cell stiffness and enhance cancer cell invasiveness. We used an anti-cancer drug, trametinib, which is an MECK kinase inhibitor. Our results revealed that trametinib treatment significantly reduce cell viscous properties but increase cell elasticity, which contribute to the decreased cell migration. These results have been published recently [3]. Via collaboration with Professor Albert Folch at the University of Washington, we further validated our studies with organotypic tissue platforms and published our findings recently [4].
To understand how cells interact with the extracellular matrix, we have recently established a traction force microscopy technique. This has been used to evaluate the effects of trametinib on cell traction forces. These results have been correlated with the cell viscoelastic properties determined via the AFM2 method in the reference [1] above, providing new, time-dependent information on cancer cell invasion. This work has been accepted as an oral presentation in the 18th International Congress on Rheology (ICR 2020).
To mimic in-vivo 3D extracellular matrix (ECM), for which we have fabricated synthetic peptide hydrogels with mechanical properties tuned to match normal and cancerous breast tissues. Culturing cells on these hydrogels allows us to study cancer cell invasion in well-controlled environments. To derive the elasticity of cells via AFM nanoindentation, mathematical models have been developed to eliminate the influence of hydrogel substrates. A manuscript is under preparation for this work.
A separate project, which links with WP2 and WP6, has been initiated with collaborators in Beatson Cancer Institute to evaluate the role of hypoxia in cancer metastasis. Results so far have provided new evidence on how hypoxia affected cell mechanical properties, cell-matrix interactions and cell migration. These provide a solid basis to develop a close-to-in-vivo vascular platform for further in-depth investigations. However, due to the Covid, the project has been interrupted.
Our plans for the next several months are:
We will complete two manuscripts for the work described above. We will implement the mathematical models in other matrix systems.
References
[1] Y.H. Chim, L. Mason, N. Rath, M.F. Olson, M. Tassieri and H. Yin. Probing the viscoelastic properties of cells by Atomic Force Microscopy: measuring the continuous frequency spectrum “in a step,”. Scientific reports (2018) 8:14462.
[2] Novo, D. et al. (2018) Mutant p53s generate pro-invasive niches by influencing exosome podocalyxin levels. Nature Communications, 9, 5069.
[3] D.A. Rudzka, G. Spennati, H. Yin and M.F. Olson. Migration through physical constraints is enabled by MAPK-induced cell softening via actin cytoskeleton re-organization. Journal of Cell Science (2019) 132, jcs224071.
[4] Spennati, G., Horowitz, L. F., McGarry, D. J., Rudzka, D. A., Armstrong, G., Olson, M. F., Folch, A. and Yin, H. (2021) Organotypic platform for studying cancer cell metastasis. Experimental Cell Research, 401(2), 112527.
....................................................................................................................
Mechanical Model of whole Cell (update: May 2018)
The aim of the research is to develop novel constitutive laws and multiscale models of cytoskeletal network dynamics.
Project 1: We will develop a type of free energy function to describe a whole cell by including the fibre (actin) dispersion, various filaments within the cell cytoskeleton and other constituents (e.g. the nucleus), viscoelastic properties of the cell, and the cell membrane.
Project 2: We will also extend the cell model to describe how mechanical and chemical stimuli influence protein expression and thus cell functionality and Growth&Remodelling of the extracellular matrix (thorough interaction with WP2) for matrix remodelling post myocardial infarction and cancer invasion.
Team: Prof.Ogden (leader), Dr. Yin, Prof. Olson, Prof. Chaplain, Prof. Insall, Prof. Husiemer, Prof. Luo, PDRA1, PhD1
WP1 Summary
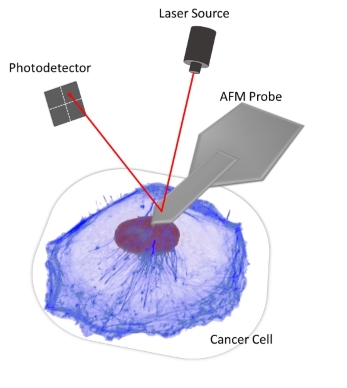
We have developed a multiscale model for an individual cell that incorporates the mechanical properties of the separate cell constituents in order to characterize the overall mechanical response of the whole cell by using a homogenization method based on volume averaging over the cell domain. The method has been applied to a prototype spherical cell consisting of a soft neo-Hookean material within a spherical shell composed of randomly distributed fibres representing the F-actin cortex. A material subroutine for a fibre-reinforced model has been implemented within the FE software FEAP. To understand the data from Atomic Force Microscopy (AFM) indentation conducted within the experimental part of the project, we have simulated a variety of simple contact problems in FEAP, with different material models and shapes of the indenters.
The experiments make use of AFM for measuring cell mechanical properties and cell-matrix interactions. Recently, we have developed a novel AFM-microrheology technique to obtain viscoelastic properties of cells and complex materials, over a wide range of continuous frequencies (0.003 Hz ~ 200 Hz), from a simple stress-relaxation nanoindentation (see figure for the a schematic of the experimental setup). Using this capability, we were able to investigate the viscoelastic responses of cells in association with cancer cell invasion. In addition, we are developing peptide hydrogels with tunable chemical and mechanical properties. This will allow further studies to be carried out in controlled 3D environments that mimic natural extracellular matrix.